NextDose: A web-based Bayesian dose forecasting tool
Last updated 17 June 2024
Gentamicin, Tobramycin,
Amikacin and Vancomycin
Target Concentration
Gentamicin
Aminoglycoside
targets are murkily defined (Matthews,
Kirkpatrick et al. 2004). But it is reasonable to consider
there are two target concentrations for gentamicin. An acceptable range for
peak concentration after the first dose is 10 to 15 mg/L (target of 12.5 mg/L).
This initial target is based on in vitro studies which indicate that
most of the bacterial killing effect can be attributed to the first dose (Barclay, Begg
et al. 1994). The risk of toxicity associated
with this concentration is considered small because of the short duration of
high concentrations and the saturable uptake mechanism for aminoglycosides into
the cochlear hair cells (Hiel, Erre et al. 1993) and renal
tubules (Barclay, Kirkpatrick et al.
1999). Vestibular toxicity is more often recognized (Ahmed, Hannigan
et al. 2012), however, reversible hearing loss
occurs and is associated with higher exposure and higher trough concentrations
of gentamicin (Abdel Jalil,
Hawwa et al. 2013).
The second
target is an average steady state concentration of 3 mg/L with an acceptable
range of 2.4 to 3.75 mg/L (Matthews,
Kirkpatrick et al. 2004). This target is equivalent to a 24
hour AUC of 72 m/L*h. Higher initial peak concentrations and 24 h AUC have been
recommended (Health
Queensland 2018).
Intermittent
24 h dosing is widely used. This minimizes ototoxicity and renal toxicity
despite high peak concentrations (see above) but is effective even though
trough concentrations at 24 h may be undetectable in patients with good renal
function. Figure 1
shows first dose predictions using a steady state target of 3 mg/L and a dosing
interval or 24 h in a 30 y old 70 kg man with normal
renal function for his weight and age. The steady state concentration at 8 h
after the dose is 2 mg/L and at 24 h after the dose is 0.4 mg/L. The initial
peak concentration is close to the target of 12.5 mg/L.


Figure 1
Tobramycin
In children with cystic fibrosis the target peak concentration of tobramycin is 30 mg/L (acceptable range 24-38 mg/L). A 24 h target AUC of 100 mg/L*h has been recommended (acceptable range 80 mg/L*h to 125 mg/L*h) (Hennig, Norris et al. 2008).
Amikacin
Exposure targets are hard to find in the literature. The target concentration for amikacin is also not clearly defined. A peak concentration target with an acceptable range of 45-60 mg/L (Health Queensland 2018) has been suggested which leads to peak concentration target value of 52.5 mg/L. Amikacin targets are typically around 3 times bigger than gentamicin so a possible target is 3 times the average steady state target of 3 mg/L which might be rounded up to 10 mg/L. An average steady state target of 10 mg/L (24 h AUC 240 mg/L*h) and a 24 h dosing interval achieves a peak within the acceptable range in a 30 y old 70 kg man with normal renal function with a 24 h trough concentration of 1.1 mg/L (Figure 2).
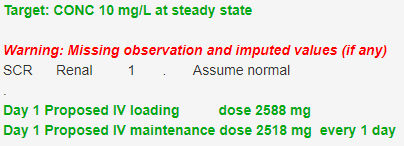

Figure 2
Vancomycin
The recommended target for vancomycin is a steady state 24 h AUC of 515 mg/L*h (average steady state target of 21.5 m/L) with an acceptable range of 400 to 600 mg/L*h (Rybak, Le et al. 2020). This AUC target (AUCssDI) achieves a peak of 68 mg/L with a 24 h trough concentration of 4.2 mg/L in a 30 y old 70 kg man with normal renal function if the dosing interval is 24 h (Figure 3).
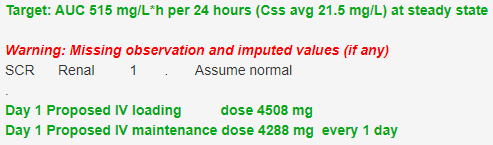

Figure 3
The guideline AUC target for vancomycin is defined by an integration interval of 24 h. However, the dosing interval for vancomycin is more commonly 12 h which leads to a different profile.
If you select the target type option “mg/L*h (AUCssDI)” then this means the AUC value you enter will depend on the chosen dosing interval (DI). Occasionally users struggle with the guideline 400 mg/L*h over 24 h AUC target when the actual (or planned) dosing interval is not 24 h. if you plan to dose every 12 h you need to half the target value to 200 mg/L*h in order to get to the guideline target of 400 mg/L*h over 24 h.
There is another option in the NextDose drop down list of target types. It now includes “mg/L*h (AUCss24)” with a default value of 400. If you use this option then you do not need to change the AUC value if you have used (or plan to use) a dosing interval such as 12 h. You can specify any dosing interval you want and NextDose will then figure out the dose for that dosing interval to achieve the steady state AUC target of 400 mg/L*h over 24 h. You may, of course, change the target value e.g. to 600 with your choice of dosing interval and the proposed dose will achieve the steady state AUC target of 600 mg/L*h over 24 h.
This AUC target of 257.5 mg/L*h over 12 h achieves a peak of 38.6 mg/L with a 12 h trough concentration of 8.8 mg/L in a 30 y old 70 kg man with normal renal function if the dosing interval is 12 h (Figure 4).


Figure 4
Note that the steady state average concentration (21.5 mg/L) is the same for both 24 h and 12 h dosing intervals. The calculation of the AUC appropriate for the dosing interval can be simply avoided by specifying the average steady state concentration (CssAvg) and entering the proposed dosing interval.
NextDose Model
The use of
TCI for gentamicin, amikacin and vancomycin has been widely used in clinical
practice for a long time. The clinical benefits of measuring concentrations are
generally accepted but without evidence from a randomized concentration
controlled trial to show that TCI is effective for these antibiotics.
A
pharmacokinetic model has been developed from a pooled data analysis of
gentamicin, amikacin and vancomycin. A joint analysis of data for these three
drugs was undertaken with the assumption that there are common features
influencing the pharmacokinetics and that sources of predictable and
unpredictable variability may be similar.
The key
differences for the gentamicin, amikacin and vancomycin models are the total
clearance, the fraction of total clearance accounted for by differences in
renal function and the volume of distribution. The residual unidentified
variability in concentrations is drug specific.
In the GAV 2020 model, clearance is based on a combination of non-renal function predicable clearance and renal function predictable clearance. The GAV 2023 model does not have a non-renal function clearance component but has a GFR associated clearance a non-GFR associated clearance, both of which change with renal function (Holford, O'Hanlon et al. 2024).
Both of these clearance components use normal fat mass which is a combination of fat free mass and a fraction of fat mass (similar to the adjusted body weight method often recommended for dosing). The PK parameters determining distribution (central volume, peripheral volume and inter-compartmental clearance) are also based on normal fat mass.
The model
shares common size components for all 3 drugs based on theory based allometry
and normal fat mass.
Maturation
of clearance, predictable from renal-function and not predictable from renal
function, use sigmoid functions of post-menstrual age with different parameters
for renal and non-renal maturation.
An
empirical decrease in non-renal function clearance with adult age was estimated
from gentamicin data and assumed to be similar for vancomycin and amikacin is
used in the 2021 GAV model. The GAV 2023 model does not have a non-renal
function clearance so there is no additional effect of adult age on clearance
In the GAV
2020 model, the changes in volume of distribution after birth use the same
exponential decrease model but with drug specific parameters describing the
magnitude and time course of approaching the adult size standardized values.
The GAV 2023 model has drug specific maturation with separate time courses and
magnitudes for central and peripheral volumes of distribution (Holford,
O'Hanlon et al. 2024)..
NextDose calculates the loading dose which achieves the same peak concentration after the first dose as that predicted at steady state from the maintenance dose and dosing interval. The loading dose depends on all the PK parameters. The principle is to achieve as rapidly as possible a concentration profile similar to that which will eventually be obtained at steady state.
The
gentamicin version of the model and parameters is used for tobramycin
calculations because the PK of gentamicin and tobramycin are essentially the
same (Regamey, Gordon
et al. 1973).
References
Abdel Jalil, M. H., A. F. Hawwa, P. J. McKiernan, M. D. Shields and J.
C. McElnay (2013). "Population pharmacokinetic and pharmacogenetic
analysis of tacrolimus in paediatric liver transplant patients." British
Journal of Clinical Pharmacology: n/a-n/a.
Ahmed, R.
M., I. P. Hannigan, H. G. MacDougall, R. C. Chan and G. M. Halmagyi (2012).
"Gentamicin ototoxicity: a 23-year selected case series of 103
patients." Medical Journal of Australia 196(11): 701-704.
Barclay, M.
L., E. J. Begg and K. G. Hickling (1994). "What is the evidence for
once-daily aminoglycoside therapy?" Clinical Pharmacokinetics 27(1):
32-48.
Barclay, M.
L., C. M. Kirkpatrick and E. J. Begg (1999). "Once daily aminoglycoside
therapy. Is it less toxic than multiple daily doses and how should it be
monitored?" Clinical Pharmacokinetics 36(2): 89-98.
Health
Queensland (2018). "Aminoglycoside dosing in adults https://www.health.qld.gov.au/__data/assets/pdf_file/0019/713323/aminoglycoside-guidelines.pdf."
Hennig, S.,
R. Norris and C. M. J. Kirkpatrick (2008). "Target concentration
intervention is needed for tobramycin dosing in paediatric patients with cystic
fibrosis – a population pharmacokinetic study." British Journal of
Clinical Pharmacology 65(4): 502-510.
Hiel, H.,
P. Erre and C. Aurousseau (1993). "Gentamicin Uptake by Cochlear Hair
Cells Precedes Hearing Impairment during Chronic Treatment." Audiology 32(1):
78-87.
Holford,
N., C. J. O'Hanlon, K. Allegaert, B. Anderson, A. Falcão, N. Simon, Y.-L. Lo,
A. H. Thomson, C. M. Sherwin, E. Jacqz-Aigrain, C. Llanos-Paez, S. Hennig, L.
Mockus and C. Kirkpatrick (2024). "A physiological approach to renal
clearance: From premature neonates to adults." British Journal of Clinical
Pharmacology 90(4): 1066-1080.
Matthews,
I., C. Kirkpatrick and N. Holford (2004). "Quantitative justification for
target concentration intervention--parameter variability and predictive
performance using population pharmacokinetic models for aminoglycosides." Br
J Clin Pharmacol 58(1): 8-19.
Regamey,
C., R. C. Gordon and W. M. Kirby (1973). "Comparative pharmacokinetics of
tobramycin and gentamicin." Clin Pharmacol Ther 14(3): 396-403.
Rybak, M.
J., J. Le, T. P. Lodise, D. P. Levine, J. S. Bradley, C. Liu, B. A. Mueller, M.
P. Pai, A. Wong-Beringer, J. C. Rotschafer, K. A. Rodvold, H. D. Maples and B.
M. Lomaestro (2020). "Therapeutic monitoring of vancomycin for serious
methicillin-resistant Staphylococcus aureus infections: A revised consensus
guideline and review by the American Society of Health-System Pharmacists, the
Infectious Diseases Society of America, the Pediatric Infectious Diseases
Society, and the Society of Infectious Diseases Pharmacists." Am J Health
Syst Pharm 77(11): 835-864.
Copyright All rights
reserved | Developed by Sam Holford & Nick Holford 2012-2024